Vein-to-vein blood bank management improving patient safety
Reduce errors and improve workflow efficiency
Designed to provide greater efficiency and avoid duplication by integrating fully with remote blood storage devices through bi-directional interfaces to all major BT
Designed by transfusionists for transfusionists
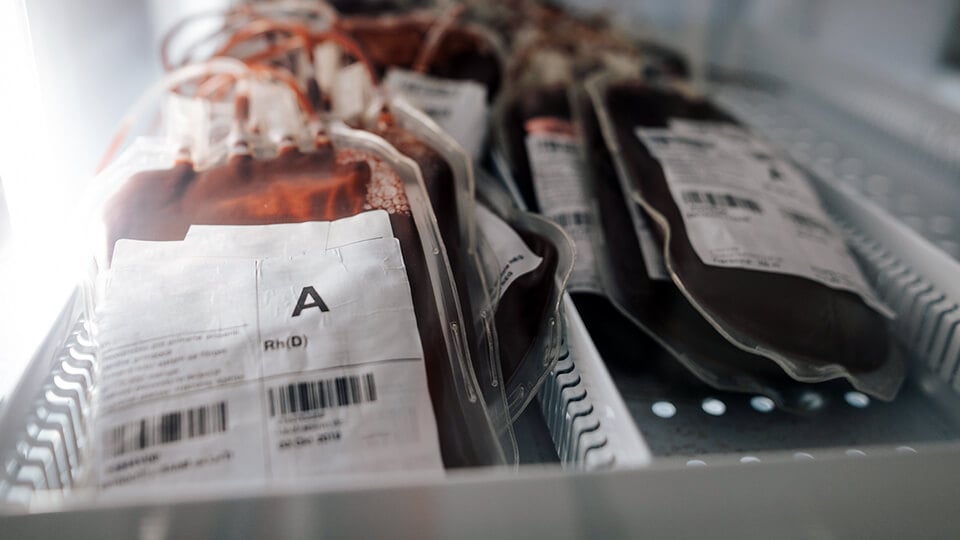
Enabling laboratory compliance
Manufactured under ISO 13485 quality management system and 510(k) authorized by the US FDA, Sunquest Blood Bank and Blood Donor, now marketed as Clinisys Blood Bank Management, enables ISO 15189 and ISO 17025 support for your laboratory systems. Key features include an integral rules base, comprehensive audit trail, and barcode-enabled throughout.
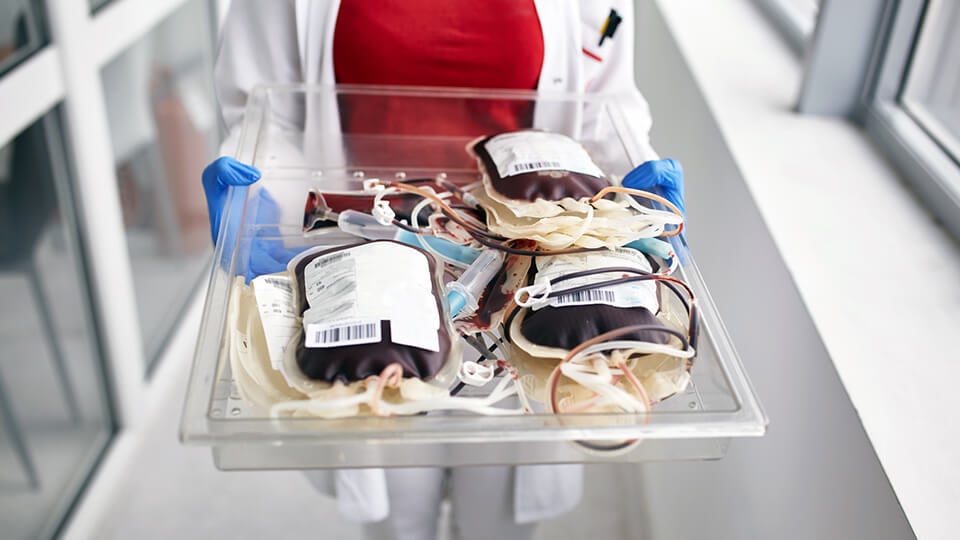
Stock management
Extensive stock management options with a proven interface to NHSBT allowing stock entry using EDN (Electronic Delivery Note). All current products issued by NHSBT are fully supported.
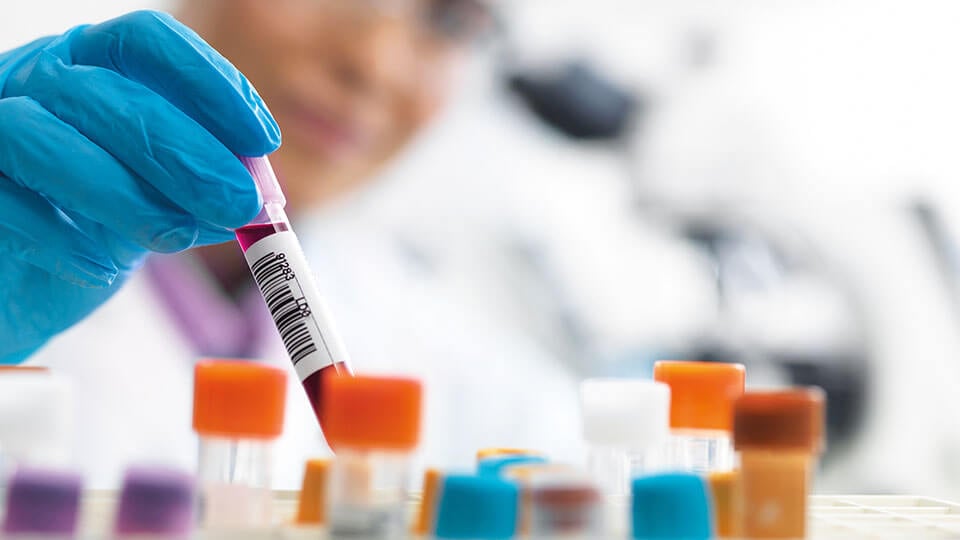
Blood transfusion statistics
A full management reporting solution provides easy access and reporting to help deliver all essential BT outputs. A variety of pre-defined operational, statistical, and management reports are provided including:
• Product turnaround times
• Unit usage
• Crossmatches performed
Laboratory solutions
Clinisys Clinical Laboratory
Clinisys Anatomic Pathology Laboratory
Clinisys Digital Pathology Management
Clinisys Microbiology Laboratory
Clinisys Molecular Laboratory
Clinisys Toxicology Laboratory
Clinisys Order & Results Management
Get in touch with us
If you’re interested in finding out more about how we can help support you in transforming your laboratory and meet your